In Salt What Is The Nature Between Sodium And Chlorine
Olivia Luz

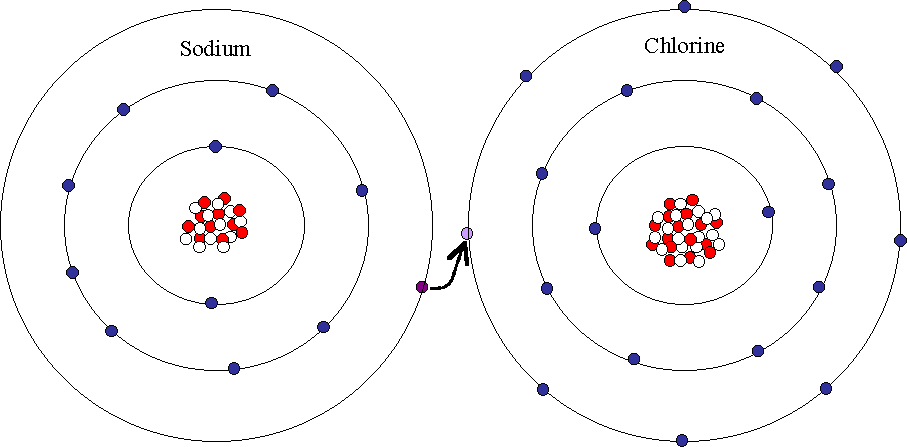
The sodium atom loses an electron and becomes a positively charged ion or cation with a charge of 1.
As the electronic transfer occurs both atoms will achieve more stable confirmations. The compound composed of these ions exhibits properties entirely different from the properties of the elements sodium and chlorine. The chlorine atom receives the electron and. The vigorous reaction between the elements sodium and chlorine forms the white crystalline compound sodium chloride common table salt which contains sodium cations and chloride anions figure figure 4 3.
The sodium na atom transfers one electron to the chlorine cl atom so that they both have full outer shells. This is because salt contains both sodium and chlorine so when salt dissociates into its ions the mass is divided not equally between sodium and chlorine ions. When sodium and chlorine atoms come together to form sodium chloride nacl they transfer an electron. Asked jan 19 2019 in class x science by muskan15 3 443 points acids bases and salts.
Get your answers by asking now. An aqueous solution of the salt ammonium chloride is acidic in nature while an aqueous solution of sodium chloride is neutral. Ionic because sodium and chlorine ions are attracted to each other. What is the result of the animated process.
RELATED ARTICLE :
- what can you eat if you have an ulcer
- what can you use in place of evaporated milk
- what can you substitute for milk in mac and cheese
In salt what is the nature of the bond between sodium and chlorine. The bond between sodium and chlorine is ionic. The end result will be a less reactive compound. The reason salt isn t just half sodium and half chlorine is because a sodium ion and a chlorine ion don t weight the same amount.
Magnesium has two electrons in its outer shell and chlorine has seven electrons in its outer shell how are the electrons transferred between these atoms asked by adminstaff 20 10 2019 09 08 pm chemistry. Ask question 100. With molar masses of 22 99 and 35 45 g mol respectively 100 g of nacl contains 39 34 g na and 60 66 g cl. Polar attractions are.
Source : pinterest.com