What Is The Number Of Valence Electrons In Cadmium Cd
Camila Farah
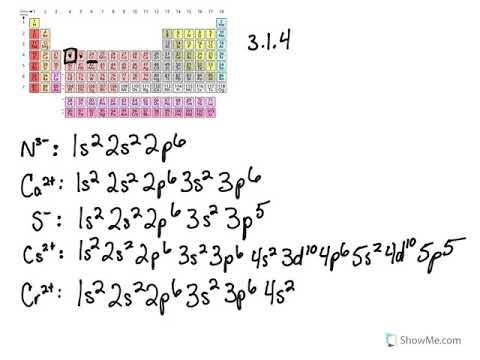
Which is the noble gas notation for lead pb.
The electron configuration of nitrogen n is. This means that it has two electrons located in its outer shell. The number of valence electrons is 2. Which of these elements has an electron configuration of kr 5s2.
Bolivianouft and 12 more users found this answer helpful 5 0 4 votes. Some are hard to memorise or predict so what is the electron configuration of an atom of cd. How many electrons does cadmium have. Cadmium cd has exactly 2 valence electrons.
The number of valence electrons in cadmium ion 48 while that of neutrons is 66. Barium calcium rubidium strontium. See full answer below. Jd3sp4o0y and 106 more users found this answer helpful.
RELATED ARTICLE :
- what is the difference between a centipede and a millipede
- what is the difference between a bug and an insect
- what is the classification of igneous rocks based on
Atomic mass of cadmium atomic mass of cadmium is 112 411 u. What is the number of valence electrons in cadmium cd. These two electrons are easily lost making a. Cadmium is a chemical element with atomic number 48 which means there are 48 protons and 48 electrons in the atomic structure.
In the case of cadmium the abbreviated electron configuration is kr 4d10 5s2. Rn 6s24f145d106p2 rn 6s25d106p2 xe 6s24f145d106p2 xe 6s25d106p2. Cadmium has 48 electrons. Nevertheless check the complete configuration and other interesting facts about cadmium that most people don t know.
The chemical symbol for cadmium is cd.
Source : pinterest.com



















